
Diversified healthcare company OPKO Health Inc. (NASDAQ: OPK) shares may be low priced, but its businesses pipeline of treatments and upside potential may deserve a higher valuation. Shares trade at 1.6X sales. OPKO owns one of the nation’s largest diagnostic laboratories, BioReference Health, which is still bleeding red after an unprecedented surge in revenues during the pandemic from COVID-19 testing.
Normalization has kicked in as sales fell precipitously. OPKO sells diagnostic test products in addition to its FDA-approved drug Rayaldee, for the treatment of secondary hyperparathyroidism for patients with stage 3 and stage 4 kidney disease. OPKO has a rich biotechnology pipeline of treatments in various stages of studies. The company is as much of an asset play in healthcare than it is a recovery play in the market.
Rich Portfolio Pipeline
OPKO built up a strong pipeline of drugs thanks to its decade-long acquisition binge rolling up many biotechs since 2012. In addition to the EBV vaccine drug MDX-2201, it also has obesity and diabetes drugs in the pipeline. Its OPK88003 is a dual agonist that binds to GLP-1 and glucagon receptors.
The company has 28 patents filed for its ModeX MSTAR plug-and-play platform. The company is seeing lots of interest for multi-specific antibodies and vaccines. It expects to close up to two transactions this year.
The ModeX MSTAR platform helps develop drugs that target up to six different biological pathways into a single molecule helping improve effectiveness in treatments for solid tumors, hematologic malignancies and leukemia. OPKO even has a NYSE: MRK">veterinary product line OPKO Health Ibero American out of Spain, which is expanding into France and European markets.
Merck: Epstein-Barr Virus Deal
OPKO Health-owned ModeX Therapeutics agreed to an exclusive worldwide license and collaboration deal with pharmaceutical giant Merck & Co. (NYSE: MRK) in the development of its Epstein-Barr virus (EBV) called MDX-2201. EBV is a common herpes virus also call mononucleosis or mono for short.
It’s fairly common in humans as most people will contact it during their lives. People can be infected with no symptoms, but it could develop into cancers like Burkitt lymphoma and nasopharyngeal carcinoma. EBV has no commercial treatment or cure as most existing treatments focus on relieving the symptoms.
Merck Deal Details
OPKO was paid $50 million upfront with additional milestone payments up to $872.5 million upon reaching prespecified and commercialization milestones. OPKO would also receive royalties on global net sales of MDX-2201 upon commercial launch. Merck will cover the clinical and regulatory costs and commercialization costs.
Merck has its sights aimed at challenging Moderna’s EBV vaccine. Ironically, Merck has partnerships with Moderna for other treatments outside of EBV. The upside potential of an EBV vaccine involves the possibility of mass vaccination requirements by the government. The EBV market could rival the influenza market.
FDA Approval for NGLENA and Pfizer Commercialization Partnership
OPKO has a partnership with Pfizer Inc. (NYSE: PFE) for the development and commercialization of NGLENA in the U.S. and worldwide. NGLENA sales are underway in 17 countries, including Japan, the U.K. and Germany. It’s been approved in 41 countries.
On June 28, 2023, the FDA approved the once-weekly treatment for pediatric growth hormone deficiency (GFD) known as dwarfism. The human growth hormone analog is for the treatment of kids three and older with growth failure stemming from inadequate secretion of endogenous growth hormone.
The U.S. launch is expected in August 2023. There is an estimated 4,000 to 10,000 children affect by dwarfism. It’s the first FDA-approved once-a-week treatment for pediatric GFD.
Trimming Losses
Opko Health reported its fiscal Q1 2023 earnings report for the quarter ending March 2023. The Company reported a GAAP earnings-per-share (EPS) loss of 2 cents, excluding non-recurring items, versus consensus analyst estimates for a loss of 8 cents $1.07, a 6-cent beat.
Revenues fell 27.8% YoY to $237.6 million from $329.2 million last year but still beat consensus analyst estimates of $193.35 million. Operating loss for the quarter fell to $30.6 million, down from $41.8 million in the year ago period. Net loss was $18 million or 2 cents per share, compared to a net loss of $55.4 million or 8 cents per share in the year ago period. OPKO ended the quarter with $110.8 million in cash and cash equivalents, excluding the $50 million prepayment from Merck.
Opko Health analyst ratings and price targets are at MarketBeat.
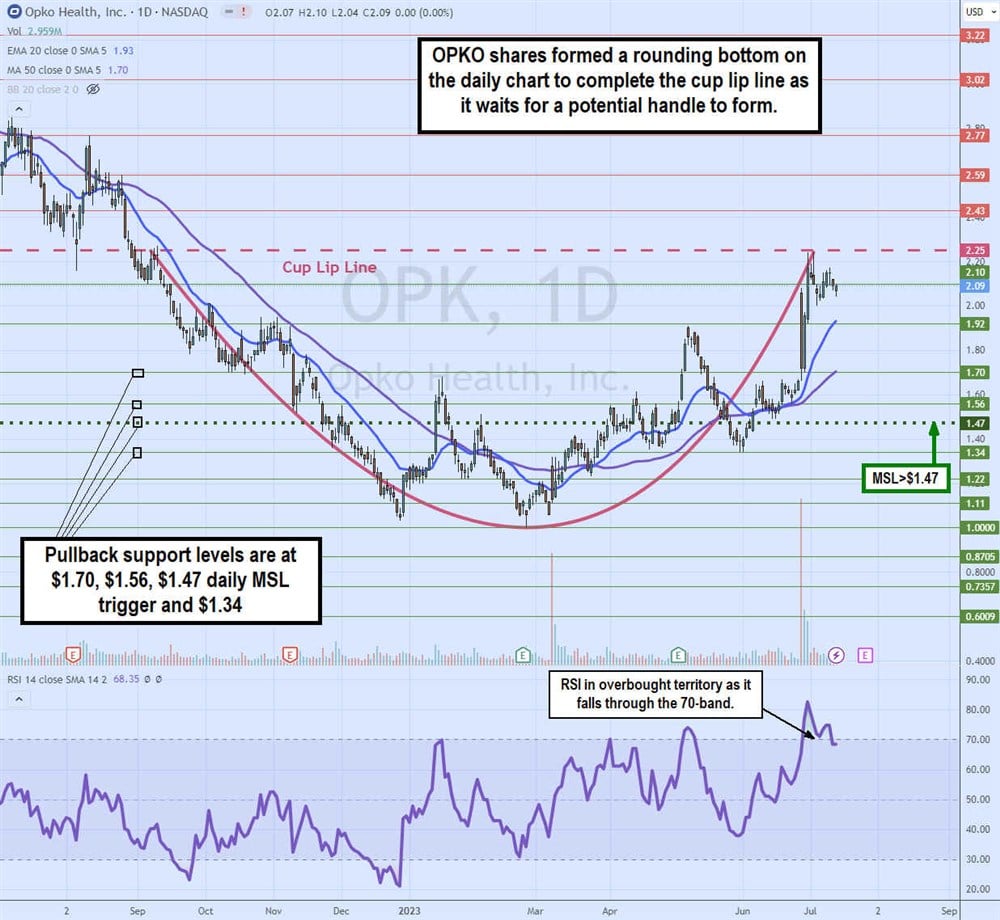
Daily Cup Preceding Potential Handle
The OPK daily candlestick chart shows the cup formation start after peaking at around $2.25 on Sept. 8, 2022. OPK proceeded to sell off to a low of $1.00 on Feb. 24, 2023. The daily relative strength index (RSI) bounced off the 30-band setting up for a fresh bounce as the rounding bottom formed.
Shares ran up towards $1.92 on May 8, 2023, before peaking and falling back to $1.34 on May 31, 2023. It formed a daily market structure low (MSL) trigger breakout through $1.47 on June 6, 2023, as shares proceeded to rally all the way back up to the cup lip line at $2.25 on July 5, 2023, before a reversion formed. The daily RSI hit the overbought 85-band on that peak and has just started to fall back under the 70-band.
Pullback support levels are at $1.70, $1.56, $1.47 daily MSL trigger and $1.34.





